|
Gut Peristalsis
The digestive system is crucial for food degestion and absorption of nutrition. The gut has several distinct redion with different functions and motility: for instance, the stomach is for digestion, and small intestine is for nutrient abdorption. The peristalsis, the wave movement of the gut, is particularly important because it conveys food to proper region in the gut at a proper timing.
Peristaltic movements are already seen in the embryonic gut, but it remains unexplored how the development of the peristalsis is regulated. We are currently studying the determination mechanisms of the origins of peristalsis,and how several types of cells (smooth muscles, interstitial cells of Cajal (ICC) and enteric neurons) are coordinated to regulate the peristalsis.
|
Vascular Network Formation
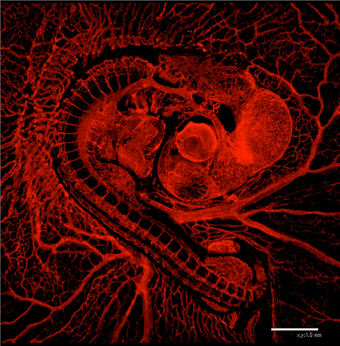
Vascular network is formed stereotypically to supply oxygen and nutrients to many organs in our body. This precise vascular patterning is established during development. We are studying how the vascular patterning is established using chicken embryos (Left panel; vascular network in E3.5 chicken embryo).
Vascular patterning in the central nervous system
In the developing central nervous system (CNS; brain and spinal cord), blood vessels grow through defined paths, establishing stereotypic patterns of vasculature. A failure of such patterning might cause a variety of CNS-associated disorders. However, the mechanisms underlying the growth/patterning of blood vessels in CNS remain poorly explored. Based on an interaction between neural tissues and vascular tissues, we are studying vascular patterning in the developing spinal cord.
Key words: Vascular patterning, CNS, Neural progenitors
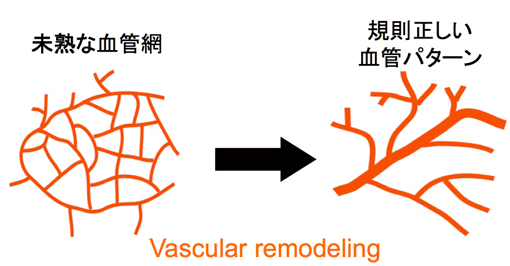
in vivo Vascular Remodeling
Many of blood vessels are initially formed as immature vascular plexus. Subsequently they change their structure to stable distinct vascular pattern (Right panel). This structure change is known as “Vascular Remodeling”. However, cellular and molecular mechanisms underlying vascular remodeling are poorly understood. We are studying vascular remodeling of chicken yolk sac vasculature using gene manipulation and live-imaging technique.
Key words: Vascular remodeling, Yolk sac vasculature, Live imaging
|
Neural crest cell
During vertebrate development, the neural crest (NC) is a transient structure that gives rise to a variety of tissues, including all types of peripheral nervous system and melanocytes in the trunk. Neural crest cells (NCCs) emigrate from the dorsal aspect of the neural tube, and migrate over distances in the body to their destinations, where they undergo terminal differentiation.
Neural crest cell migration
We focus on the migration of NCCs during development. Our research goal is to understand the mechanism underlying NCCs migration using chicken embryos and molecular approaches. We are particularly interested in the migration of NCCs of sympathetic and medullary lineages. We found that the dorsal aorta acts as a morphogenetic signaling center that coordinates NCC migration (Saito, D et al., Science, 2012).
Key words: Sympathetic lineage, Cell migration, Dorsal aorta
|
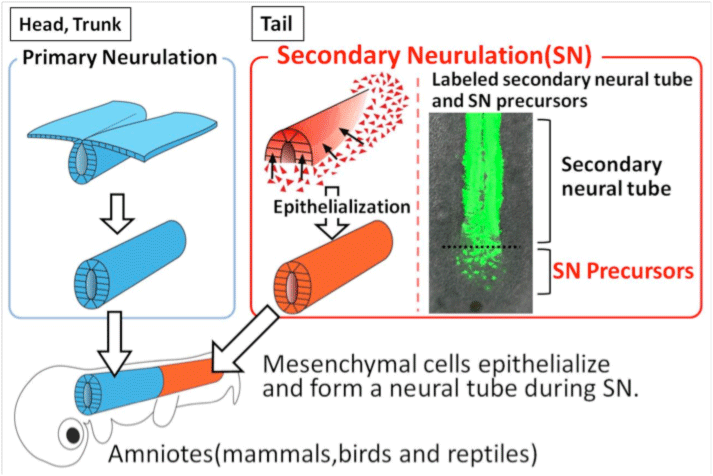
Secondary Neurulation
The neurulation in early embryogenesis is the onset of all the nervous system. In amniotes (mammals, birds, reptiles), two types of neurulation processes are seen. One is the well known neurulation where a sheet of epithelial cells folds inward to form a neural tube. Another one, called secondary neurulation (SN), is seen in the posterior region of the body, which proceeds by mesenchymal-to-epithelial transition (MET). However, the detail about the molecular and cellular mechanisms of SN remains unknown. We have developed the gene manipulate technique for SN-precursors. Now we aim to elucidate the mechanisms of SN and to understand the basic principle of the tail formation.
Key words: Secondary neurulation, Stem/progenitor cells, Tail formation
|
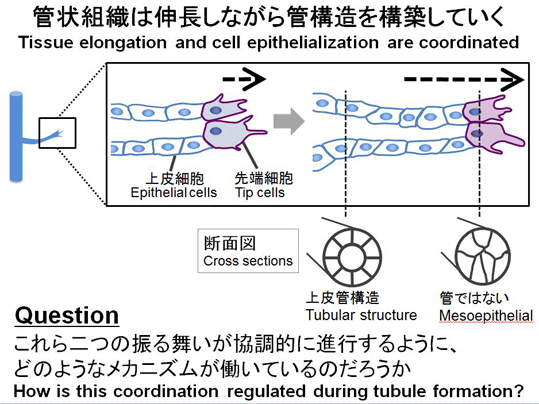
Tubulognesis using Wolffian duct as a model
Epithelial tubules play important roles in physiological functions in many organs. Although tubulogenesis involves morphologically distinct steps, i.e. tissue elongation and cell epithelialization, they must proceed in a coordinated manner in the body. To understand how such coordination is regulated, we established an experimental model using Wolffian duct of chicken embryos (WD; also called a nephric duct), the earliest basis for kidney formation. WD is a simple structure, elongating posteriorly as a straight cord. With a WD-specific gene manipulation, we performed time-lapse imaging analyses in vivo. Using this experimental system, we are asking how the processes of tubulogenesis proceed coordinately during development.
Key words; Tubulogenesis, Wolffian duct, Tissue elongation, Cell epithelialization
Epithelial-to-mesenchymal transition (EMT)
Epithelial cells play a central role in physiological functions of many organs. A failure of the epithelial integrity not only leads to physiological malfunctions, but also causes metastatic cancer by the process known as epithelial-to-mesenchymal transition (EMT). EMT has been thought to be influenced by nearby stroma, often considered as an amorphous tissue. However, the molecular and cellular entity of the stroma remains elusive. Since many organs in the actual body are filled with different types of epithelia, one type of epithelium might serve as a stroma for another type. To address these issues, we established a new experimental model in which two different epithelia are separately gene-manipulated in chicken embryos. One epithelium is Wolffian duct (“WD”, considered as a stromal component in this study), and the other is nephric coelomic epithelium (Neph-CE) overlying the stromal WD. By using this model system, we demonstrated that inter-epithelial interactions are essential for maintaining epithelial integrity, where ECM acts an important mediator.
Key word: Epithelial-mesenchymal transition, Extra cellular matrix, Cell-cell interaction
↑top
|